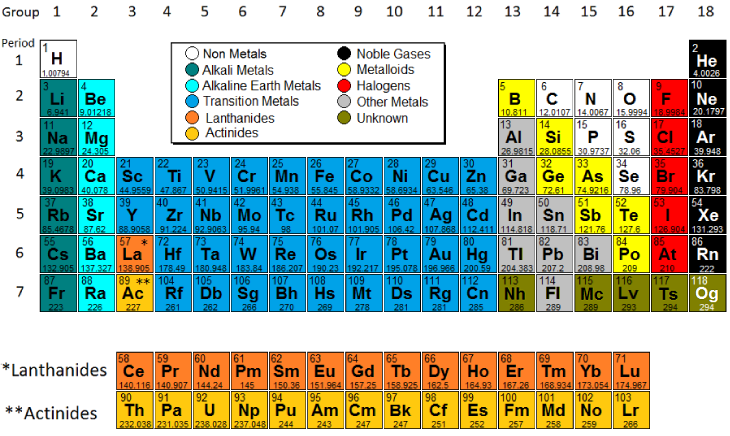
Which Are the Main Regions of the Periodic Table
The periodic table also known as the periodic table of the chemical elements is a tabular display of the chemical elementsIt is widely used in chemistry physics and other sciences and is generally seen as an icon of chemistryIt is a graphic formulation of the periodic law which states that the properties of the chemical elements exhibit a periodic dependence on their atomic. Alkali metals Alkaline earth metals Transition metals lanthanoids actinides and those labeled other metals to.
Periodic Table Model Science Software
Groups are given a number to show where they are in the periodic table and also to identify the group of elements in them.
. For the table to fit on a single page parts of two of the rows a total of 14 columns are usually written below the main body of the table. There are seven periods in the periodic table with each one beginning at the far left. A s only B p only C s or p.
Metals nonmetals gases B. They can conduct electricity but not as well as a metal conducts electricity. In fact we recommend that you introduce the periodic table in the elementary years.
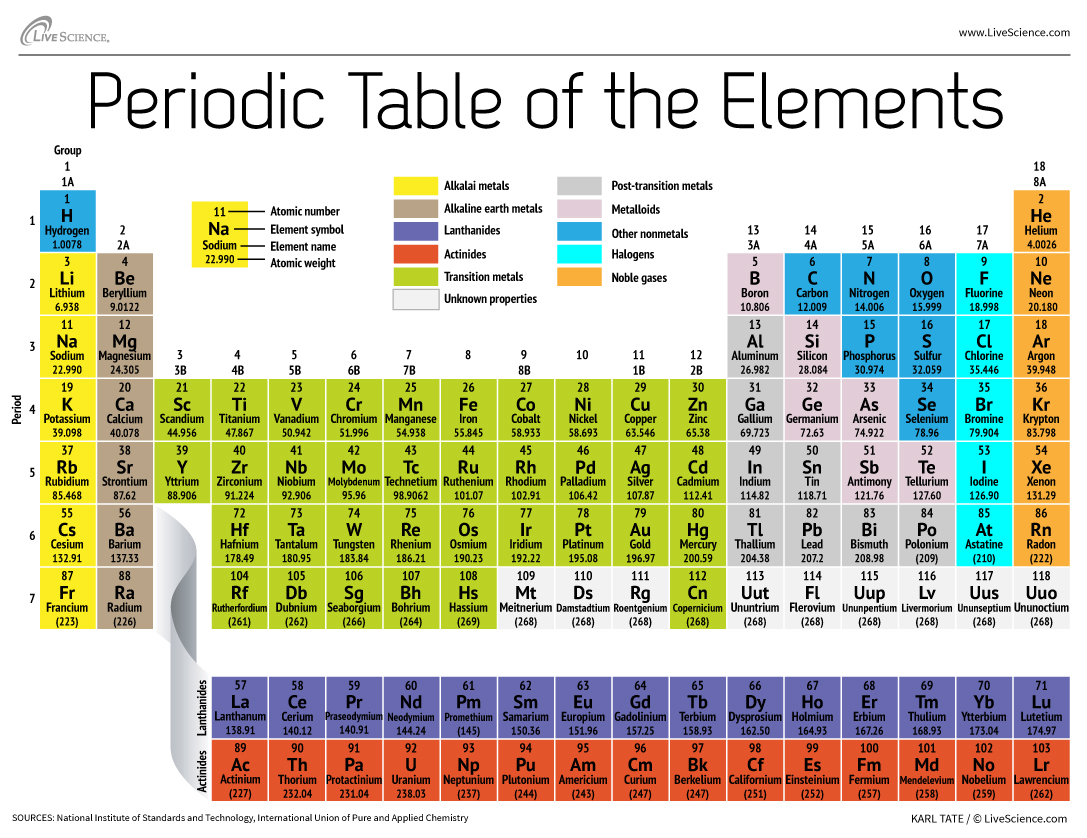
The columns of the periodic table are called groups. Alkaline earth metals group. Up to 24 cash back Nonmetals.
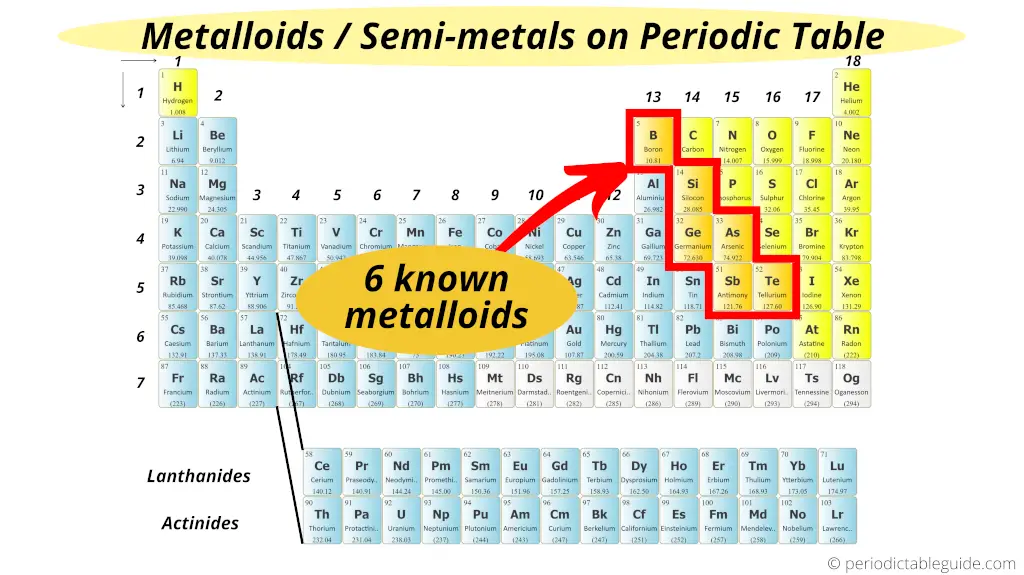
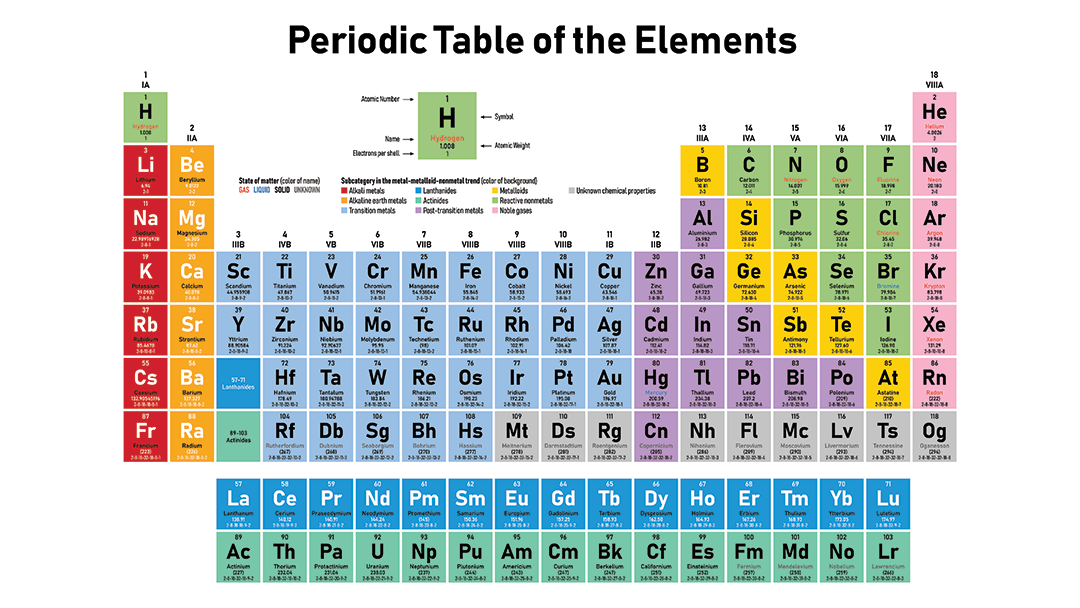
So spd f. There are three major classification as metal regions on the left of the periodic table non-metals except hydrogen on the right of the periodic table and metalloids in between the periodic table. Solids liquids gases C.
Elements in the same group have the same number of valence electrons and. And purple a metalloid. The alkali metals make up most of.
A row of the periodic table. They are found in trace amounts in the atmosphere in fact 1 of the atmosphere is argon. Group 8A or VIIIA of the periodic table are the noble gases or inert gases.
Alkali metals group hydrogen not included Group 2. Future Groups of the Periodic table Alkali metals. The periodic table today is arranged with two different parts the groups and the periods.
A column of the periodic table. Semimetals metalloids Major Regions of the Periodic Table. The periodic table has three main regionsmetals on the left nonmetals except hydrogen on the right and metalloids in between.
The Periodic Table - A Brief Explanation of one of the Foundations of Chemistry. Transition and Inner transition metals group. Helium is also found.
Regions of the Periodic Table. Groups in Periodic Table With Group Names There are total 18 different groups in Periodic table. Elements that have similar properties are arranged in groups or families - vertical columns.
The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs. The four you need to know are s sharp p principle d diffused and f fine or fundamental. It is possible to define the periodic table elements as metals nonmetals and metalloids.
The three broad categories of elements are metals nonmetals and metalloids. Our current periodic table lists 109 elements. Major Regions of the Periodic Table.
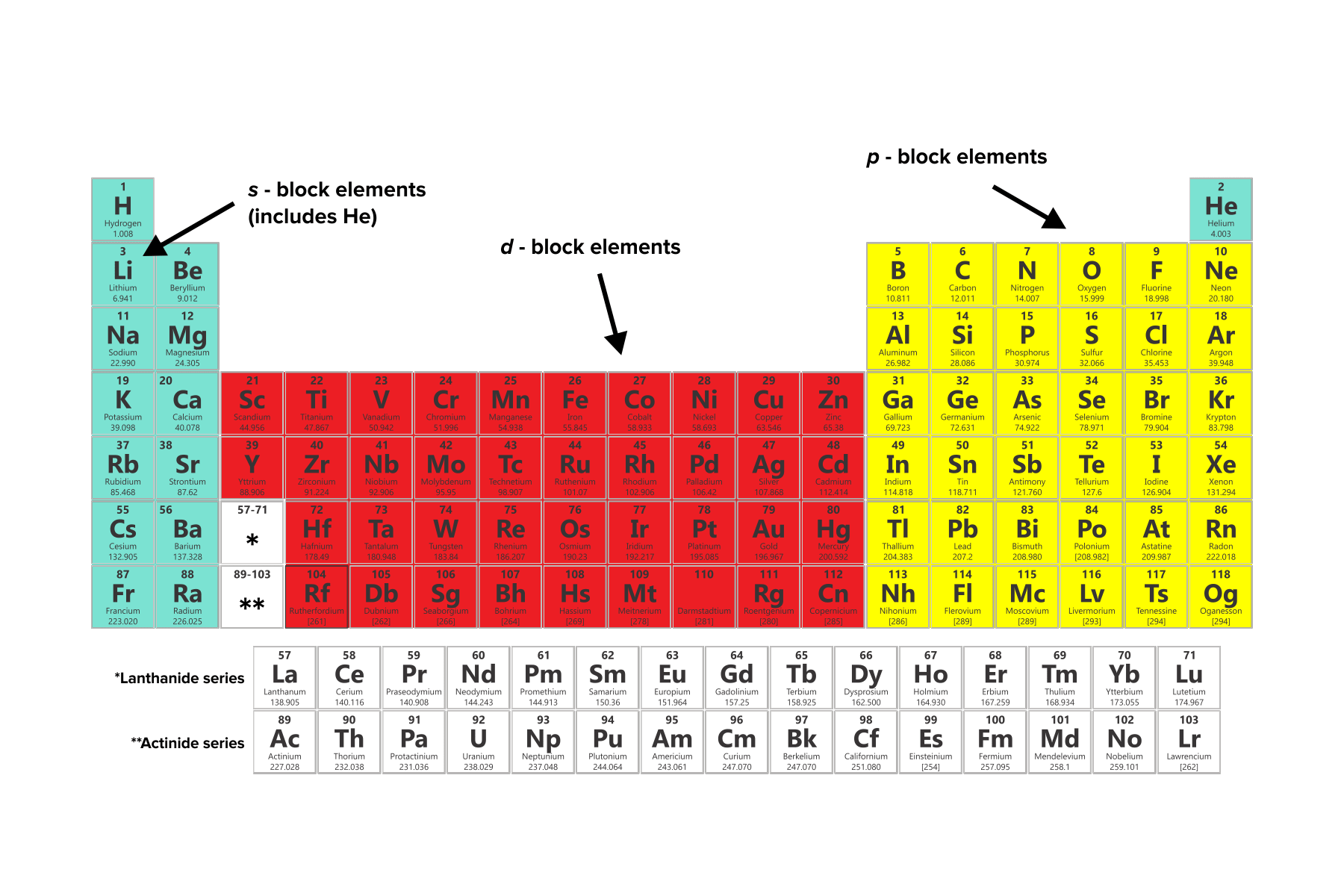
Position on the periodic table indicates the properties of its element. Based on electron configurations the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. In the regions of the periodic table associated with the main group elements which type of orbitals are being filled.
The shorter groups the transition metals are numbered from IB1B through VIIIB 8B. It is arranged according to the periodic law. The Group 18elements Located in the nonmetalregion.
Reading the Periodic Table. It is a key concept in chemistry that needs to be taught to our students. There are four regions on the periodic table the s p d and f blocks which refers to the highest energy orbitals being filled with electrons.
The atomic number of selenium is 34 which places it in period 4 and group 16. 2nd largest region poor conductors. All elements in a group share the same number of valence electrons.
Traditionally in the United States the taller groups on the table the main group elements are numbered from IA1A through VIIIA8A. Located bt metals and nonmetals Have properties of both metals AND nonmetals Good semiconductors. However IUPAC recommends that the numbers 1 through 18 be used and these labels are more common.
Properties of the elements are periodic meaning that they repeat after a specific interval. Elements are arranged in order of increasing atomic number their physical and chemical properties show a periodic pattern. 1 and then classify the element according to its location.
A new period begins when a new principal energy level begins filling with electrons. LAST SUB LEVEL CONFIGURATION Each sublevel is assigned a letter. The groups are the vertical columns.
Which regions on the periodic table are metals. Metalloids have properties of both metals and nonmetals. A yellow box indicates a metal.
Elements in the periodic table are organized according to their properties. Period 1 has only two elements hydrogen and helium while periods 2 and 3 have 8 elements. INTRODUCTION THE PERIODIC TABLE OF ELEMENTS CAN BE DIVIDED INTO FOUR REGIONS OR BLOCKS.
There are four regions on the Periodic Table the s p d and f blocks which refers to the highest energy orbitals being filled with electrons. The periodic table visually shows the elements that make up everything we see around us. Mendeleev put elements with similar properties and that react in similar ways into the same.
Nonmetals are located on the righthand side of the periodic table. The periodic table of elements is arranged into several broad groups Image credit. 1 selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals so.
Elements in the same period have their highest energy electrons in the same principal energy level. Most elements are metals. Which are the main regions of the periodic table.
Find selenium in the periodic table shown in Figure 25. Helium He neon Ne argon Ar krypton Kr xenon Xe and radon RnThe name comes from the fact that these elements are virtually unreactive towards other elements or compounds. Largest region conduct heat and electricity lustrous shiny ductile can draw into wire and malleable can bend and be hammered.
The s p d and f blocks are illustrated below. A period is a horizontal row of the periodic table. The peri-odic table on pages 20 and 21 indicates these regions with different colors.

The Periodic Table Of Elements Biology For Majors I
Groups And Periods Of The Periodic Table Metals Nonmetals And Metalloids Elements And The Periodic Table
Where Are Metalloids Located On The Periodic Table Images

Group Definition Facts Britannica
Atoms And Periodic Trends For The Mcat Everything You Need To Know Shemmassian Academic Consulting
(215).jpg)
Parts Of The Periodic Table Quiz Test Proprofs Quiz
How The Periodic Table Groups The Elements Live Science

Properties Of Chemical Elements Britannica
Scientists Say Periodic Table Science News For Students

Periodic Table Of The Elements Stock Illustration 54766997 Pixta

How To Read The Periodic Table Groups Periods Chemtalk

1 The Periodic System Of The Elements Reference Download Scientific Diagram

Periodic Table Of The Elements Stock Illustration 45043394 Pixta
Groups And Periods Of The Periodic Table Metals Nonmetals And Metalloids Elements And The Periodic Table

How To Write Electron Configurations For Atoms Of Any Element Electron Configuration Periodic Table Chemistry Help


Comments
Post a Comment